39 what is an open label study
Open label study | definition of open label study by Medical dictionary open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. An Open-label, Phase I/II Study of the Pan-immunotherapy in Patients ... An Open-label, Phase I/II Study of the Pan-immunotherapy in Patients With Relapsed/Refractory Ovarian Cancer. The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government.
What is an open label extension study? • NCK Pharma An open - label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered.
What is an open label study
Open-Label Trial - an overview | ScienceDirect Topics An open-label single-dose trial evaluated safety, pharmacokinetics, sensory gating, attention, impulsivity, and inhibition in 12 adult males and females. The single dose of fenobam was associated with a significant improvement of PPI compared to the untreated control group from a previous study (Berry-Kravis et al., 2009 ). medical-dictionary.thefreedictionary.com › open-label+studyOpen-label study | definition of open-label study by Medical... open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc. clinicalinfo.hiv.gov › en › glossaryOpen-Label Trial | NIH - HIV.gov Open-Label Trial A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants. Related Term (s) Clinical Trial Double-Blind Study
What is an open label study. Facebook - National Cancer Institute NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine. Reducing bias in open-label trials where blinded outcome ... - NCBI Nov 21, 2014 ... Many trial designs do not permit blinding, and are therefore designed as open-label, with patients, clinicians, and other study investigators ... Open-Label Trial - Vial An open-label trial is a clinical trial design where there is no blinding or masking implemented, and investigators and trial subjects are aware of the ... › understanding-clinical-trialUnderstanding Clinical Trial Terminology: What is a Long-Term... An OLE study is a clinical trial that typically enrolls participants of a previous clinical trial and is designed to gather the long-term safety and tolerability data on a potential new medicine beyond the time period of the main study.
jcto.weill.cornell.edu › open_clinical_trials › an-open-labelAn Open-Label Study to Assess the Anti-Tumor Activity and Safety... This clinical trial is for men and women with B-cell Non-Hodgkin Lymphoma (NHL). The purpose of this study is to study is to assess the safety and effectiveness of odronextamab in destroying participant's cancer cells. Odronextamab is an investigational drug, which means it has not yet been approved by the U.S. Food and Drug Administration (FDA). Open-label Definition & Meaning - Merriam-Webster open-label: [adjective] being or relating to a clinical trial in which the treatment given to each subject is not concealed from either the researchers or the subject — compare double-blind, single-blind. What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials can be used to gather additional safety and efficacious data on drugs on the market to increase the confidence of clinicians, patients, and clinical bodies. They can play a... pubmed.ncbi.nlm.nih.gov › 17253876Open-label extension studies: do they provide meaningful ... - ... Open-label extension studies do have a legitimate but limited place in the clinical development of new medicines. The negative perceptions about these studies have arisen because of perversion of acceptable rationales for this type of study and a failure to recognise (or disclose) the limitations resulting from the inherent weaknesses in their ...
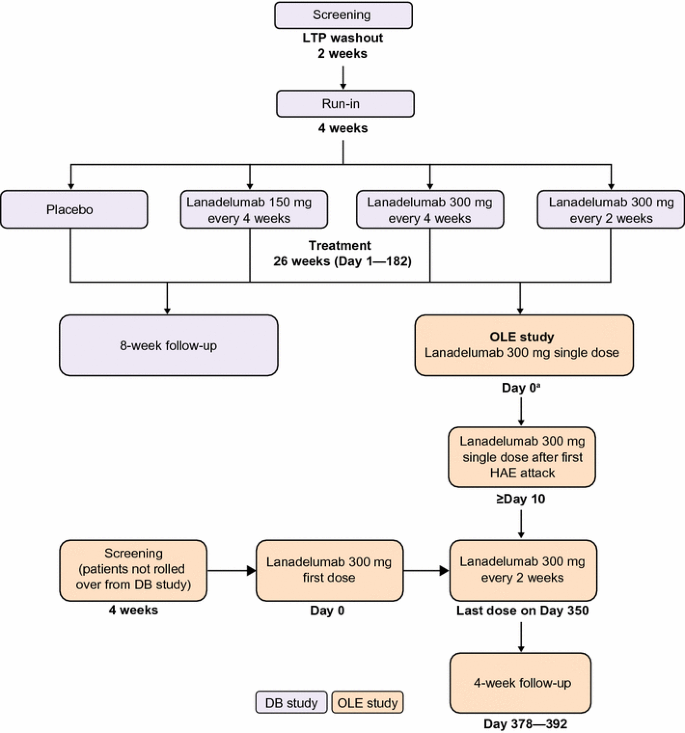
dian.wustl.edu › our-research › clinical-trialEnd of Trial and Open-Label Extension (OLE) Frequently Asked... Open Label Extension, or OLE, is a phase of a study that occurs after the randomized (blinded) portion of the trial is completed if a drug is found to have the potential for benefit. Eligible trial participants take the active form of the drug without placebo. OLE allows active drug to be given to all participants at the same time and to follow them over time. Open Label Study to Evaluate Efficacy and Long Term Safety of LUM001 ... This is an open label study in children with Progressive Familial Intrahepatic Cholestasis (PFIC) designed to evaluate the safety and efficacy of LUM001, also known as Maralixibat (MRX). Efficacy will be assessed by evaluating the effect of LUM001 on pruritus and the biochemical markers of pruritus associated with PFIC. What is an Open Label Study in Clinical Research? | Power In an open label study, or open label trial, all participants know exactly what treatment/intervention they will be receiving in the study. Study physicians and investigators also know what each participant is receiving. That means that there is no uncertainty regarding whether or not you may be receiving a placebo rather than the study treatment. › understanding-clinical-trialUnderstanding Clinical Trial Terminology: What is an Open Label... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population.
Open-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1]
Open Clinical Trials | Bioclever Blog Dec 10, 2020 ... A trial is open or open label when the patient knows if he/she has been assigned to the experimental or control treatment arm. · A single-blind ...
FDA Guidance: "Design Considerations for Pivotal Clinical ... One objective of a clinical study: eliminate, reduce or estimate the bias, so as to characterize in a accurate manner the safety and effectiveness of the device Variance can be reduced by a more...
Orelabrutinib for the treatment of relapsed or refractory MCL ... - PubMed Orelabrutinib for the treatment of relapsed or refractory MCL: a phase 1/2, open-label, multicenter, single-arm study Blood Adv. 2023 Apr ... Patients with r/r MCL were enrolled in this phase 1/2 study and treated with orelabrutinib, a novel, highly selective BTK inhibitor. The median number of prior regimens was two (range, 1 to 4). The median ...
An Open Label, Randomized, Multicenter Study of Elafibranor ... oral elafibranor at 2 doses (80 and 120mg) in children 8-17 years and 2. assess changes in aminotransferases. Methods: Children with NASH were randomized to open-label elafibranor 80mg or 120mg daily for 12 weeks. The intent-to-treat analysis included all participants who received at least one dose. Standard descriptive statistics and PK analyses were performed. Results: Ten males (mean 15 ...
Medical Definition of Open-label - MedicineNet Open-label: A term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving. CONTINUE SCROLLING OR CLICK HERE SLIDESHOW
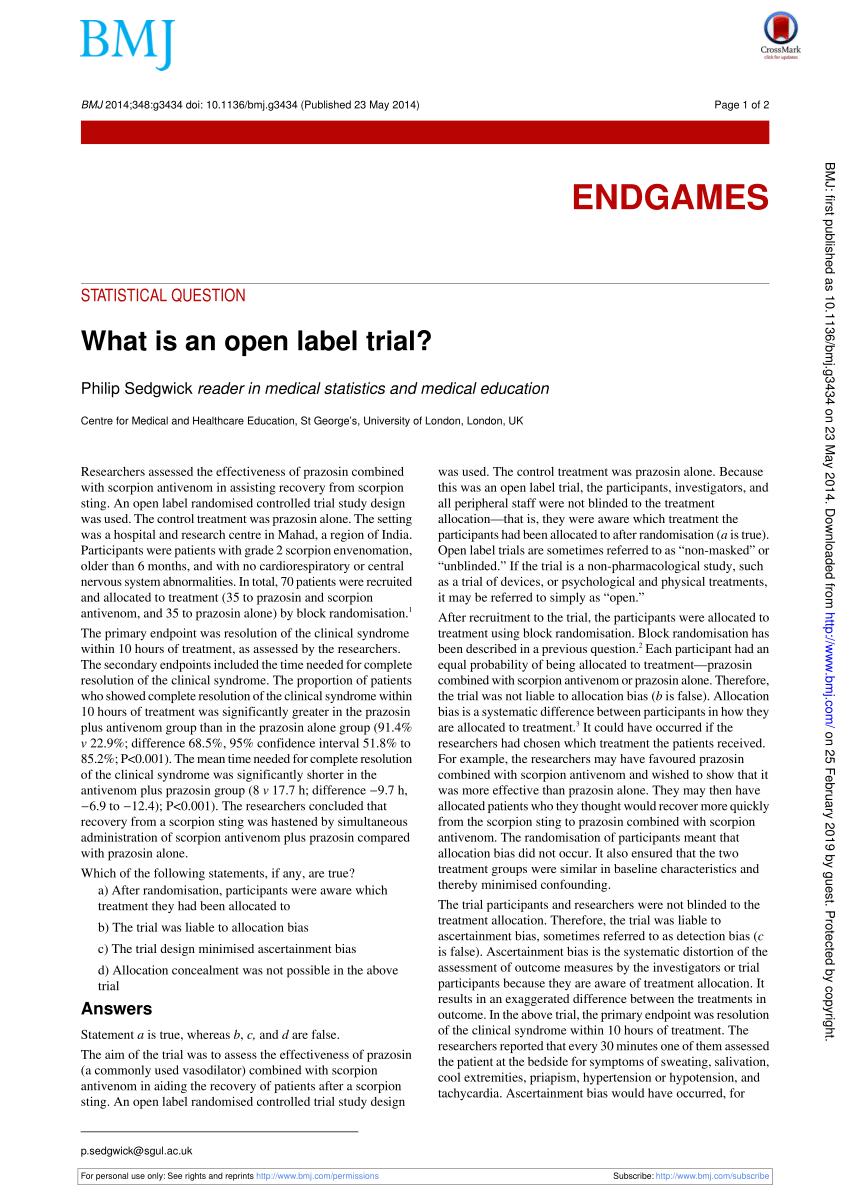
(PDF) What is an open label trial? - ResearchGate An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India....
Open-Label Trial - an overview | ScienceDirect Topics Open-label extension studies: In these studies, which often follow a double-blind randomized placebo-controlled trial, subjects have the option of remaining on the study intervention in an open-label fashion (i.e., they know that they are on the study intervention) for an extended period of time (e.g., several years). They may be informed of ...
open-label trial - Traduction française - Linguee Open-label trial-A clinical trial in which [...] researchers and participants know which drug or vaccine is being administered. myeloma-euronet.org. myeloma- ...
PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology factors. In the case of open-label extension studies, we feel that analysis of efficacy ought to regard treatment allocation as a possible explanatory variable (in addition to others, such as baseline status and time). The study by Mease and colleagues 1 reports the results of a 48-week open-label extension of a 24-week randomized
What is an open label trial? | The BMJ An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants were patients with grade 2 scorpion envenomation, older than 6 months, and with no cardiorespiratory or central nervous system abnormalities.
Open-Label Extension Studies | SpringerLink As the name implies, an open-label extension study is an 'appendage' to a randomised controlled clinical trial, usually of an unregistered medicine or intervention. Often the drug is being studied under an investigational new drug (IND) licence or equivalent legislation. The open-label extension study is identified formally as a study.
clinicalinfo.hiv.gov › en › glossaryOpen-Label Trial | NIH - HIV.gov Open-Label Trial A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants. Related Term (s) Clinical Trial Double-Blind Study
medical-dictionary.thefreedictionary.com › open-label+studyOpen-label study | definition of open-label study by Medical... open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc.
Open-Label Trial - an overview | ScienceDirect Topics An open-label single-dose trial evaluated safety, pharmacokinetics, sensory gating, attention, impulsivity, and inhibition in 12 adult males and females. The single dose of fenobam was associated with a significant improvement of PPI compared to the untreated control group from a previous study (Berry-Kravis et al., 2009 ).








![PDF] Bias was reduced in an open-label trial through the ...](https://d3i71xaburhd42.cloudfront.net/814ad6ccbfa9defca4d2b00c4672f9070cf6b8da/16-Figure1-1.png)

















Komentar
Posting Komentar